Details of the Drug
General Information of Drug (ID: DMZ6VEY)
| Drug Name |
Sulfacetamide
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
Acetocid; Acetosulfamin; Acetosulfamine; Albamine; Albucid; Alesten; Cetamide; Formosulfacetamide; Klaron; Oclucid; Ophthacet; Region; SULSTER; Sebizon; Solfacetamide; Steramide; Sulamyd; Sulfacet; Sulfacetamida; Sulfacetamidum; Sulfacetimide; Sulfacil; Sulfacyl; Sulfacylum; Sulfair; Sulfanilacetamide; Sulfanilazetamid; Sulphacetamide; Sulphacetamidum; Sulphasil; Sultrin; Urosulfon; Urosulfone; Isopto cetamide; Sodium sulamyd; Sodium sulfacetamide; Solfacetamide [DCIT]; Sulfacetamide Monosodium Salt; Sulfacetamide Sodium Usp; Sulfacetamide sodium; Sulfacetamide sodium anhydrous; Sulfanilazetamid [German]; Sulphacetamide sodium; Sulfair 15; A-500; Ak-Sulf; Bleph-10; Bleph-10 liquifilm; FML-S; I-Sulfacet; Isopto-Cetamide; Klaron (TN); N-Acetylsulfanilamide; N-Acetylsulfanilamine; N-Sulfanilylacetamide; N-Sulphanilylacetamide; N1-Acetylsulfanilamide; OP-Sulfa 30; Ocusulf-10; Ophthel-S; P-Aminobenzenesulfonacetamide; P-Aminobenzenesulfonoacetamide; Steri-Units Sulfacetamide; Sulf-10; Sulf-15; Sulfacel-15; Sulfacetamida [INN-Spanish]; Sulfacetamide [USAN:INN]; Sulfacetamidum [INN-Latin]; Sulfacyl (VAN); Sulfair-15; Sulten-10; N'-Acetylsulfanilamide; N(sup 1)-Acetylsulfanilamide; N(sup1)-Acetylsulfanilamide; Sulfacetamide (USP/INN); N-(4-Aminobenzenesulfonyl)acetamide; N-(4-Aminophenylsulfonyl)acetamide; N-(4-aminophenyl)sulfonylacetamide; N-Acetyl-4-aminobenzenesulfonamide; N-[(4-Aminophenyl)sulfonyl]acetamide; N-[(p-Aminophenyl)sulfonyl]acetamide; N(sup 1)-Acetyl-4-aminophenylsulfonamide; N-((4-Aminophenyl)sulfonyl)acetamide; N-((p-Aminophenyl)sulfonyl)acetamide
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Indication |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Therapeutic Class |
Antibiotics
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Affected Organisms |
Enteric bacteria and other eubacteria
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| ATC Code | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure |
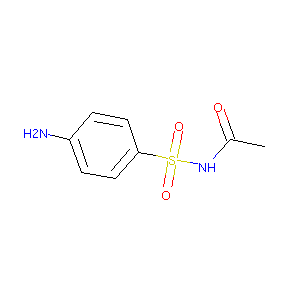 |
|||||||||||||||||||||||||||||||||||||||||||||||||||
| 3D MOL | 2D MOL | |||||||||||||||||||||||||||||||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 214.24 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 4 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Identifiers |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||
| Cross-matching ID | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Repurposed Drugs (RPD) | Click to Jump to the Detailed RPD Information of This Drug | |||||||||||||||||||||||||||||||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | |||||||||||||||||||||||||||
Drug Inactive Ingredient(s) (DIG) and Formulation(s) of This Drug
